
An Anterior Cruciate Ligament Injury Prevention Framework: Incorporating the Recent Evidence (2012)
C. J. Donnellya, B. C. Elliotta, T. R. Acklanda, T. L. A. Doylea, T. F. Beiserb, C. F. Finchc, J. L. Cochraned, A. R. Dempseyae & D. G.
Lloydaf*
Research in Sports Medicine: An International Journal
Volume 20, Issue 3-4, pages 239-262
Abstract
Anterior cruciate ligament (ACL) injury rates have increased by ∼50% over the last 10 years. These figures suggest that ACL focused research has not been effective in reducing injury rates among community level athletes. Training protocols designed to reduce ACL injury rates have been both effective (n = 3) and ineffective (n = 7). Although a rationale for the use of exercise to reduce ACL injuries is established, the mechanisms by which they act are relatively unknown. This article provides an injury prevention framework specific to noncontact ACL injuries and the design of prophylactic training protocols. It is also apparent that feedback within this framework is needed to determine how biomechanically relevant risk factors like peak joint loading and muscular support are influenced following training. It is by identifying these links that more effective ACL injury prevention training programs can be developed, and, in turn, lead to reduced ACL injury rates in the future.
INTRODUCTION
Anterior cruciate ligament (ACL) ruptures are severe sport injuries, dramatically affecting an athlete’s ability to return to play following reconstruction (Dunn & Spindler, 2010; Roos, Ornell, Gardsell, Lohmander, & Lindstrand, 1995). Furthermore, following an ACL rupture, when accompanied by a meniscal injury, the probability that an athlete will develop radiographic diagnosed knee osteoarthritis (OA) within 10 to 15 years increases by 20–50% (Oiestad, Engebretsen, Storheim, & Risberg, 2009).
In the United States, ACL injury estimates prior to 1998 were 23/100,000 people per year, increasing to 35/100,000 people per year in 2006 (Lyman et al., 2009; The World Bank, 2010). These U.S. figures are consistent with current estimates from both New Zealand (2000–2005) and Scandinavia (2004–2007), which have reported ACL injury rates of 32–37/100,000 (Gianotti, Marshall, Hume, & Bunt, 2009) and 38/100,000 (Granan, Forssblad, Lind, & Engebretsen, 2009) people per year, respectively. In Australia (2003–2008), ACL injury rates have been reported to be as high as 52/100,000 people per year (Janssen, Orchard, Driscoll, & van Mechelen, 2011). Improved injury surveillance, increases in sport participation and exposure, or rule changes within a sport to increase the speed of play may all have contributed to the observed increases in ACL injury estimates. However, with such a large increase (∼ 50%) over such a short period of time (∼ 10 years), it is apparent that in the context of the community level athlete, ACL injury prevention research is not being effectively translated into injury prevention practice.
Training interventions designed to reduce ACL injury rates in general athletic populations have been shown to be both effective (Caraffa, Cerulli, Projetti, Aisa, & Rizzo, 1996; Hewett, Lindenfeld, Riccobene, & Noyes, 1999; Mandelbaum et al., 2005), and ineffective (Heidt, Sweeterman, Carlonas, Traub, & Tekulve, 2000; Junge, Rosch, Peterson, Graf-Baumann, & Dvorak, 2002; Myklebust et al., 2003; Pfeiffer, Shea, Roberts, Grandstrand, & Bond, 2006; Soderman, Werner, Pietila, Engstrom, & Alfredson, 2000; Steffen, Myklebust, Olsen, Holme, & Bahr, 2008; Wedderkopp, Kaltoft, Holm, & Froberg, 2003). Although empirical research has shown that balance, plyometric, and/or neuromuscular training can be used to reduce ACL injury rates, the mechanisms by which such training protocols act is still relatively unknown, which is evident from the large number of ACL injury prevention studies with inconclusive findings.
Ultimately, the mechanism of an ACL injury is that the loads applied to the ligament are greater than its ability to sustain the load (Lloyd, 2001). All ACL injury prevention programs, whether designed for males or females, should therefore focus on reducing the loads applied to the joint and in turn ACL during sporting tasks. The loads applied to the ACL are influenced by externally applied joint loads, the activation of muscles capable of supporting these loads, the orientation of the tibiofemoral joint when loads are applied, as well as the anatomical alignment of the ligament. The focus of this review is on interventions designed to reduce external joint loads and/or improve muscular support during noncontact sporting tasks.
Training interventions must be designed to target the causal factors associated with ACL injury (Lloyd, 2001) if positive treatment effects can be effectively translated to, and adopted by, community level athletes (Finch, 2006). It is beyond the scope of this article to describe the epidemiology of ACL injuries and the evidence for their risk factors in detail. Rather, this article will present a framework for translating ACL focused research into injury prevention practice in the context of the community level athlete. Through the development of this framework, a scientific rationale for the design of ACL injury prevention training protocols will also be presented.
ACL INJURY PREVENTION FRAMEWORK
The six stage injury prevention model to Translate Research into Injury Prevention Practice (the TRIPP model) proposed by Finch (2006) provides a blueprint for preventing injuries in sport. Borrowing from the TRIPP model, this article provides an ACL injury prevention framework specific to, and detailed for, the intrinsic factors associated with noncontact ACL injuries and an empirically based rationale for the design of ACL injury prevention training protocols.
Injury Surveillance
General population estimates show 32–52/100,000 people per year rupture their ACL, with the majority occurring during sport (Gianotti et al., 2009; Granan et al., 2009; Lyman et al., 2009; Janssen et al., 2011). Retrospective surveys (Gianotti et al., 2009; Rochcongar, Laboute, Jan, & Carling, 2009) and video analyses of athletes rupturing their ACL (Cochrane, Lloyd, Buttfield, Seward, & McGivern, 2007; Krosshaug et al., 2007) show that approximately half occur during noncontact situations. Of these noncontact injuries, almost all occur during landing or sidestepping, immediately after foot contact, with the knee near full extension (Cochrane et al., 2007; Koga et al., 2010; Krosshaug et al., 2007). Further classification of noncontact landing injuries shows that the majority occur during single-leg landing situations (Cochrane et al., 2007; Koga et al., 2010).Mechanical Aetiology of ACL Injury
The ultimate mechanism of an ACL injury is that the forces applied to the ligament are greater than its ability to sustain the load (Lloyd, 2001). Experimental laboratory, in-vivo/cadaveric and in-silico research have provided valuable information to better understand what loading patterns, joint kinematics, and phases of movement are associated with increased ACL injury risk. Using this information, a model for the aetiology of ACL injuries can be formulated and, in turn, appropriate countermeasures developed.
Valgus, internal rotation knee moments and anterior tibial translations relative to the femur (anterior drawer) all elevate ACL strain in cadaveric knee models (Markolf et al., 1995; Shin, Chaudhari, & Andriacchi, 2011). However, it is the combined loading of these moments/forces that contributes to the largest ACL strain measurements and injury risk. For example, tibiofemoral compression and internal rotation moments (Meyer & Haut, 2008), valgus and internal rotation moments (Shin et al., 2011), and anterior drawer combined with either valgus or internal rotation moments (Markolf et al., 1995) all elevate ACL strain more than anterior drawer alone. Simulation studies (in-silico) support the view that anterior draw alone is not the likely mechanism of ACL injury; the addition of valgus knee moments are required to achieve injurious loads (McLean, Huang, Su, & Van Den Bogert, 2004; McLean, Huang, & van den Bogert, 2008).
Laboratory-based analyses of noncontact sidestepping have shown that compared with straight-line running, peak extension knee moments are similar, while internal rotation and/or valgus knee moments are up to two-times higher (Besier, Lloyd, Ackland, & Cochrane, 2001; Besier, Lloyd, Cochrane, & Ackland, 2001; Dempsey et al., 2007). Valgus and internal rotation knee moments are also elevated during single-leg landing (McLean, Borotikar, & Lucey, 2010; McLean & Samorezov, 2009). Hewett et al. (2005) showed valgus knee moments observed during double-leg landing can predict the ACL injury status of adolescent females with 73% specificity and 78% sensitivity. Again, these are the same loading patterns shown to elevate ACL strain in cadaveric knee models (Markolf et al., 1995; Shin et al., 2011). It should be noted that peak internal rotation and/or valgus knee moments are elevated further when sidestepping (Besier, Lloyd, Ackland, et al., 2001) and single-leg landing (McLean et al., 2010) are performed in unplanned situations.
Peak in-vivo ACL strain measured in a single healthy male has been shown to occur during the weight acceptance (WA) phase of stance (first 20%–30%) during the deacceleration phase of a landing task (Cerulli, Benoit, Lamontagne, Caraffa, & Liti, 2003), which are similar to the accelerations observed during the WA phase of sidestepping (Jindrich, Besier, & Lloyd, 2006). Additionally, WA is where peak internal rotation and/or external valgus knee moments are observed during sidestepping (Besier, Lloyd, Ackland, et al., 2001; Besier, Lloyd, Cochrane, et al., 2001; Cochrane et al., 2010; Dempsey, Lloyd, Elliott, Steele, & Munro, 2009; Dempsey et al., 2007) and single-leg landing (McLean et al., 2010). It is therefore logical to identify the WA phase of landing and sidestepping as when the ACL is at greatest risk of injury.
Knee valgus angle or “dynamic valgus” angle during double-leg landing has been shown to be significantly greater in ACL injured versus uninjured adolescent female populations and a predictor of ACL injury (R2 = 0.88; Hewett et al., 2005). It should be appreciated however, that knee range of motion in the varus/valgus degree of freedom is limited and unlikely to reach a spread of 30° across participants as reported previously (Hewett et al., 2005). Measurements of “dynamic valgus” angles are, to a certain extent, projections resulting from a combination of femoral internal rotation and knee flexion, which is likely the reason that the reliability of knee varus/valgus (median CMC = 0.74) joint angle measurements are substantially lower than knee flexion/extension (median CMC = 0.96) joint angle measurements (McGinley, Baker, Wolfe, & Morris, 2009). It is acknowledged that “dynamic valgus” knee postures are indeed associated with ACL injury risk (Hewett et al., 2005). However, the means by which athletes attain these postures is likely due to poor hip neuromuscular control during WA, which has been shown to be associated with peak frontal, saggittal, and/or transverse knee loading during both sidestepping (McLean, Huang, & van den Bogert, 2005) and single-leg landing (Kipp, McLean, & Palmieri-Smith, 2011).
Knee flexion angle is another factor that affects the transfer of external knee loads to the ACL (Fukuda et al., 2003; Markolf et al., 1995; Wu, Seon, et al., 2010). The ACL consists of two bundles, the anteromedial bundle (AMB) and posterolateral bundle (PLB), named from their insertions on the tibial plateau. Direct strain measures of the AMB and PLB in a cadaveric knee model have shown these bundles function in a reciprocal manner, with the PLB taut in extension (0°–15°) and the AMB taut in flexion (60°–90°; Gabriel, Wong, Woo, Yagi, & Debski, 2004). However, when quadriceps muscle loads are simulated, the functioning of the AMB and PLB change and begin working in a complementary manner, with the peak strain of both bundles observed near full extension (i.e., 0 and 15° of knee flexion; Wu, Seon, et al., 2010). Modelling of the AMB and PLB during the stance phase gait (0.7 m/s) has shown the kinematics of the ACL change as a function of knee flexion angle, with peak elongation observed near full extension (Wu, Hosseini, et al., 2010). These results support a mechanistic rationale as to why most noncontact ACL injuries occur with the knee close to full extension (Cochrane et al., 2007; Koga et al., 2010; Krosshaug et al., 2007).
It is clear that increasing knee flexion can reduce ACL strain and hip neuromuscular control is associated with peak knee loading and ACL injury risk. The role of the muscles in supporting the hip and knee during sporting tasks, however, should not be overlooked. As the knee flexes, the moment arms of the muscles crossing the knee joint change, altering their ability to support external knee loads (Lloyd & Buchanan, 2001). When the knee is flexed, the hamstring muscles are aligned to resist anterior drawer, while the lateral hamstrings are capable of better supporting internal rotation moments (Buford, Ivey, Nakamura, Patterson, & Nguyen, 2001). Conversely, the medial hamstrings and quadriceps both become less capable of supporting valgus knee moments (Lloyd & Buchanan, 2001). When the knee is extended, the opposite is true. Further research is needed to determine how knee kinematics and the hip and trunk musculature influence knee loading during sporting tasks. With more sophisticated modelling techniques future research may be able to develop subject-specific models capable of quantifying the complex interaction between hip kinematics, knee flexion angle, muscle force estimates, knee joint loading, and ultimately ACL strain to allow for a better assessment of how muscles function to support the knee and mitigate ACL strain and injury risk during sporting tasks.
Countermeasures
One logical method to reduce the risk of ACL injury would be to strengthen the tissue itself, making it more capable of withstanding larger loads. Following a period of immobilization, mobilization (i.e., exercise) can be used to stimulate collagen regeneration in rabbit medial collateral ligamentous tissues and Rhesus monkey ACL tissues to 79% (Woo et al., 1987) and 91% (Noyes, 1977) of the strength of comparable healthy tissues. Surprisingly, to our knowledge, no published peer-review study has shown that training can be used to promote collagen regeneration that leads to significant strength increases in healthy ACL tissues. In fact, research has shown that post maturation, collagen concentration and ligament force tolerance in healthy ACL tissues significantly decrease with age (Amiel, Kuiper, Wallace, Harwood, & VandeBerg, 1991; Noyes & Grood, 1976). This provides a rationale to focus on reducing the loads applied to the ACL. Two approaches can be used to reduce ligament loading: (1) change an athlete’s technique during a sporting task to reduce external joint loading and (2) increase the strength and/or activation of the muscles supporting the knee and ACL when external joint loading is elevated.
Technique and Knee Loading
The potential for upper body segments to influence the loading of distal joints in the kinematic chain is substantial. Over one-half of a person’s mass is located in the head, arms, and trunk, which are located over one-half of an individual’s total height from the ground (Winter, 2005). Hip neuromuscular control (McLean et al., 2005), lateral trunk flexion (Dempsey et al., 2007), and restraining an athlete’s arm close to midline (Chaudhari, Hearn, & Andriacchi, 2005) have all been shown to increase valgus and/or internal rotation knee moments during sidestepping. Hip neuromuscular control has also been shown to be the primary predictor of both frontal and transverse plane knee loading during single-leg landing (Kipp et al., 2011).
Altering a person’s technique during sidestepping has been proven effective in reducing valgus knee moments during sidestepping (Dempsey et al., 2009). The three recommendations made to athletes were to place their stance foot closer to the body’s midline, while keeping their torso upright and rotated toward the desired direction of travel (Dempsey et al., 2009). Motor control strategies to reduce external knee loading during single-leg landing tasks have not yet been tested.
Identifying direct, causative links between an athlete’s kinematics and joint loading is difficult when treating sidestepping and single-leg landing as multisegment, dynamic movements. As such, limited causal information linking critical aspects of the movement pattern to the knee loading patterns associated with elevated ACL injury risk is available. Future research is needed to establish these causal links if more refined and effective ACL injury prevention training protocols can be developed in the future.
Neuromuscular Support
There is no single muscle crossing the knee capable of simultaneously supporting the knee from externally applied flexion, valgus, and internal rotation knee moments. For this reason, multiple muscle activation strategies can be used to reduce ACL injury risk during sidestepping and single-leg landing. When simulating the contact phase of landing in a cadaveric knee model, Hashemi et al. (2010) found that increased quadriceps force in the precontact (PC) phase of landing resulted in lower ACL strain during the impact phase. The reductions in ACL strain were attributed to the quadriceps’ ability to prevent the tibia from translating relative to the femur by both increasing joint stiffness at low knee flexion angles and producing posteriorly directed joint reaction forces past 20° of knee flexion (Hashemi et al., 2010). These results are supported by Wu, Seon, et al. (2010) who has shown that the application of a 400 N quadriceps force can reduce peak AMB force by almost 50% (123N to 75N).
Due to their line of action, hamstring muscle force can reduce ACL tension from 15° to 45° of knee flexion (More et al., 1993). Anterior cruciate ligament (ACL) strain is reduced further however, when the hamstrings are cocontracted with the quadriceps (Withrow, Huston, Wojtys, & Ashton-Miller, 2008). The cocontraction of the quadriceps and hamstring muscle groups reduces ACL tension from 15°–60° of knee flexion by resisting the displacement of the tibia relative to the femur in all three planes of motion (Li et al., 1999; Withrow et al., 2008). Valgus and internal rotation knee moments can be supported with the activation of specified muscles crossing the knee joint (Lloyd, Buchanan, & Besier, 2005). Generally, medial knee muscles have moment arms capable of supporting valgus knee moments (Buchanan & Lloyd, 1997; Lloyd & Buchanan, 1996, 2001; Lloyd et al., 2005) and considered an appropriate neuromuscular strategy for supporting the knee and ACL from external valgus knee moments (Buchanan & Lloyd, 1997; Lloyd & Buchanan, 2001).
In summary, appropriate muscle activation strategies to counter applied flexion, valgus, and/or internal rotation knee moments during sidestepping and single-leg landing include generalized hamstring/quadriceps cocontraction, superimposed with the increased activation of muscles with flexion and/or medial moment arms.
ACL Focused Training Intervention Protocols
A review of the current ACL injury prevention literature was conducted. The databases of Science Direct, EMBASE, Web of Science, AUSport Med, Medline, and Ovid SP were searched. The search was restricted to human studies, conducted between 1990 and July 2011, and written in English. Search terms included (sprain* or injur* or rupture* or strain* or tear* or trauma* or pain* or stiff*) AND (prevent* or risk* or rate* or safe* or prophylactic*) AND (tibiofemoral* or knee* or ACL or anterior cruciate* or cruciate*). From the six databases, 2,541 titles and abstracts were assessed and reviewed. Of these, 53 manuscripts were considered further, and then 20 manuscripts were selected for inclusion in the final review. Inclusion criteria were restricted to training interventions measuring changes in ACL injury rates (n = 10), laboratory-based, biomechanically focused training interventions (n = 6) and field-based, biomechanically focused training interventions (n = 4).
For the purpose of this review, when appropriate, ACL injury prevention protocols were classified into four general categories: (1) plyometric training: exercises with ballistic movements containing both concentric and eccentric phases (i.e., jumping and landing); (2) balance training: postural exercises with an unstable base of support and/or single-leg support with or without external perturbations; (3) technique training: instructional feedback immediately prior to, following, or during a sport task (i.e., running, landing, and sidestepping); and (4) resistance training: movements performed against external forces progressively overloading isolated muscle groups.
Following a review of the literature, it was found that combinations of plyometric, balance, resistance, and/or technique training can be used to reduce ACL injury rates in athletic populations (Caraffa et al., 1996; Hewett et al., 1999; Mandelbaum et al., 2005). Similar ACL injury prevention protocols, however, have been shown to be inconclusive or ineffective in reducing ACL injury rates in general athletic populations (Heidt et al., 2000; Junge et al., 2002; Myklebust et al., 2003; Pfeiffer et al., 2006; Soderman et al., 2000; Steffen et al., 2008; Wedderkopp et al., 2003). Athlete compliance with the training protocols (Myklebust et al., 2003; Soderman et al., 2000; Steffen et al., 2008), intervention exposure (Soderman et al., 2000; Steffen et al., 2008), and/or specificity of the training intervention (Junge et al., 2002; Myklebust et al., 2003) were identified as some external factors that may have prevented the combined effects of plyometric, balance, resistance, and/or technique training from being translated into reductions in ACL injury rates. Nonetheless, these conflicting results illustrate the point that biomechanical measurements like technique, joint loading, and muscle support during sidestepping and single-leg landing need to be measured in parallel with changes in ACL injury rates. Through this approach, it may be possible to identify the biomechanical mechanisms by which training influences the factors associated with noncontact ACL injuries and why a particular training protocol led to a positive or inconclusive training outcome.
Laboratory-based, biomechanically focused training interventions (Table 1) such as plyometric, balance, resistance, and/or technique training have been proven effective in reducing peak valgus knee moments (Hewett, Stroupe, Nance, & Noyes, 1996; Myer, Ford, Palumbo, & Hewett, 2005) and increasing knee flexion angle (Myer, Ford, McLean, & Hewett, 2006; Myer et al., 2005) during preplanned double-leg (Hewett et al., 1996; Myer et al., 2006; Myer et al., 2005) and single-leg (Myer et al., 2006) landing tasks. These results demonstrate that training as a whole is effective in altering an athlete’s knee joint biomechanics and subsequent ACL injury risk. Still, it is unclear what the most appropriate training protocols or combinations of exercises should be for reducing ACL injury risk during double-leg or single-leg landing tasks.
Field-based, biomechanically focused training interventions (Table 2) have shown the combined training effects of plyometric, balance, resistance, and/or technique training, implemented adjunct to an athlete’s normal in-season training, are effective in reducing ACL injury risk during double-leglanding, double-leg stop-landing and sidestepping tasks (Chappell & Limpisvasti, 2008; Lim et al., 2009; Zebis et al., 2008). Lim et al. (2009; Y. S. Lee, personal communication, January 19, 2011) reported reductions in peak extension and valgus knee moments as well as elevated hamstring-quadriceps cocontraction during the WA phase of double-leg landing. Chappell and Limpisvasti (2008) reported reductions in valgus knee moments during a double-leg stop-landing task. Zebis et al. (2008) reported increases in medial hamstring muscle activation in the precontact phase of sidestepping. However, because external knee loading was not measured (Zebis et al., 2008), it is unclear if the observed changes in hamstring activation are in response to training or increases/changes in knee joint loading. It should also be noted that all of these studies were conducted in “ideal” training environments with high athlete compliance, which was as high as 100% (Chappell & Limpisvasti, 2008) and low coach-to-athlete ratios during training, which were approximately 3:11 (Lim et al., 2009) and 2:33 (Chappell & Limpisvasti, 2008).
When tested in isolation, resistance training was not effective in reducing external knee loading and subsequent ACL injury risk during preplanned double-leg landing or sidestepping (Cochrane et al., 2010; Herman et al., 2008). Conversely, both balance and technique training, tested in isolation, have been proven effective in reducing peak valgus (Cochrane et al., 2010; Dempsey et al., 2009) and internal rotation (Cochrane et al., 2010) knee moments during preplanned and unplanned sidestepping. Although providing more clarity as to which training interventions most influence external knee loading, again, the aforementioned training interventions were all performed under “ideal” training settings (Cochrane et al., 2010; Dempsey et al., 2009; Hewett et al., 1996; Myer et al., 2006; Myer et al., 2005).
To our knowledge, the efficacy and effectiveness of plyometric, balance, resistance, and/or technique training in reducing the biomechanical factors associated with ACL injury risk have not been tested in “real-world” settings, using a randomized control trial (RCT) design. Future research is needed to fill this gap if positive laboratory-based findings can be translated to “real-world” community level training environments. Additionally, it is important to continue to test training interventions in isolation, but also to identify the causal links between specific training classifications and surrogate biomechanical measures of ACL injury risk. From both approaches, we will be better able to develop ACL injury prevention training protocols that target the factors associated with ACL injury risk and increase the probability of transferring positive laboratory-based training effects to “real-world” training environments.
ATHLETE SCREENING
Injury prevention training programs have been shown to have greater effects on “high-risk” relative to “low-risk” athletic populations (Myer, Ford, Brent, & Hewett, 2007). The ability to use screening tools to identify “high-risk” athletic populations would provide health care professionals with the ability to develop athlete-specific ACL injury prevention training protocols. Tibial-femoral bone geometry, such as narrow intercondylar notch width and a steep posterior tibial slope angle, have both been associated with elevated ACL injury risk (Simon, Everhart, Nagaraja, & Chaudhari, 2010). The ability to image an athlete’s tibial morphology is expensive and is a nonmodifiable risk factor, limiting the use of this type of screening tool in community level athletic populations.
Clinically relevant tests for estimating ACL injury risk have shown that medial/lateral upper body stability is the strongest single predictor of ACL injury (Overall p = 0.02; Odds Ratio = 2.2) (Zazulak, Hewett, Reeves, Goldberg, & Cholewicki, 2007), while whole body postural stability is one of four predictors of ACL reinjury (C statistic = 0.94; Paterno et al., 2010). Although more accessible, cost effective, and modifiable, these clinically relevant screening tools provide limited causal information between their predictive measures and the biomechanical factors associated with ACL injury risk. If available, this information can be used to prescribe personalized injury prevention training protocols that target the biomechanics factors classifying an athlete as “high-risk” as well as maximizing the impact of a prophylactic training protocol.
SUMMARY
Figure 1 presents an injury prevention framework specific to and detailed for non-contact ACL injuries. In summary, the injury surveillance literature has shown that the majority of sport-related ACL injuries occur during noncontact sidestepping and single-leg landing tasks (Stage 1). Combined externally applied flexion, valgus, and internal rotation knee moments during the WA phase of sidestepping and single-leg landing with the knee near full extension is the likely mechanism of noncontact ACL injuries (Stage 2). Countermeasures to reduce the biomechanical factors associated with ACL injury risk should have three foci: (1) to reduce the magnitude of externally applied flexion, valgus, and internal rotation knee moments; (2) to increase muscular support against these aforementioned joint moments; and (3) to increase knee flexion angle and the neuromuscular control of the hip during the WA phase of sidestepping and single-leg landing; although the extent of (3) is still to be defined (Stage 3).
FIGURE 1 ACL injury prevention framework to translate ACL-focused research in injury prevention practice.
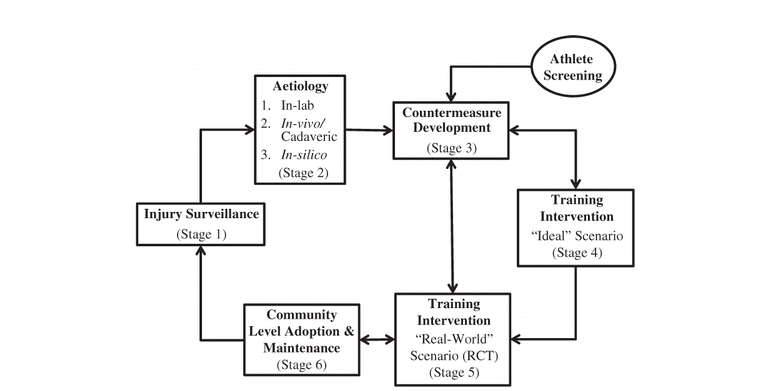
The combined effects of plyometric, balance, resistance, and/or technique training have been effective in reducing the biomechanical factors associated with ACL injury in “ideal” training environments (Stage 4). Literature testing the efficacy of ACL injury prevention protocols largely remains in stage 4. We now need to test the efficacy of these training interventions in “real-world” settings using an RCT design to determine if positive laboratory-based biomechanical training outcomes, like reducing peak knee loading and/or increasing muscular support, can be effectively translated to community level training environments (Stage 5). Future research is also needed to evaluate the challenges associate with implementing effective “real-world” training interventions within community level training environments (Stage 6). The overall goal of such evaluations will be to observe reductions in ACL injury rates across heterogeneous community level athletic populations (Stage 1). It should be noted that continuous, reliable, nationwide annual injury surveillance systems are needed before the long-term effectiveness and cost benefits of “real-world” ACL injury prevention training protocols in reducing ACL injury rates can be evaluated.
Adding to the TRIPP model (Finch, 2006), the ACL injury prevention framework includes athlete screening to identify “high-risk” athletes, allowing for the development of athlete-specific ACL injury prevention training protocols. Finally, it is evident that the use of feedback within the ACL injury prevention framework is needed to determine how biomechanically relevant risk factors like peak joint loading and muscular support are influenced following a training intervention and/or during athlete screening. By identifying these causal relationships, ACL injury prevention training programs can be created to target the biomechanically relevant factors associated with ACL injury risk in both general and “high-risk” athletic populations. It is through this approach that more effective injury prevention training programs can be developed and, in turn, ACL injury rates can be reduced in the future.
Acknowledgments
The authors wish thank the Australian National Health and Medical Research Council (project grant numbers 400937, 565907), the Australian Football Research and Development Board, the Western Australian Medical Health and Research Infrastructure Fund and The University of Western Australia for financial support. CFF is supported by an NHMRC Principal Research Fellowship (565900).
REFERENCES
1. Amiel, D., Kuiper, S. D., Wallace, C. D., Harwood, F. L. and VandeBerg, J. S. 1991. Age-related properties of medial collateral ligament and anterior cruciate ligament: A morphologic and collagen maturation study in the rabbit. Journal of Gerontology, 46(4): B159–165.
2. Besier, T. F., Lloyd, D. G., Ackland, T. R. and Cochrane, J. L. 2001. Anticipatory effects on knee joint loading during running and cutting maneuvers. Medicine & Science in Sports & Exercise, 33(7): 1176–1181. [Web of Science ®], [CSA]
3. Besier, T. F., Lloyd, D. G., Cochrane, J. L. and Ackland, T. R. 2001. External loading of the knee joint during running and cutting maneuvers. Medicine & Science in Sports & Exercise, 33(7): 1168–1175. [CrossRef], [PubMed], [Web of Science ®], [CSA]
4. Buchanan, T. S. and Lloyd, D. G. 1997. Muscle activation at the human knee during isometric flexion-extension and varus-valgus loads. Journal of Orthopaedic Research, 15(1): 11–17. [CrossRef], [Web of Science ®], [CSA]
5. Buford, W. L. Jr., Ivey, F. M. Jr., Nakamura, T., Patterson, R. M. and Nguyen, D. K. 2001. Internal/external rotation moment arms of muscles at the knee: Moment arms for the normal knee and the ACL-deficient knee. Knee, 8(4): 293–303. [CrossRef], [PubMed], [Web of Science ®], [CSA]
6. Caraffa, A., Cerulli, G., Projetti, M., Aisa, G. and Rizzo, A. 1996. Prevention of anterior cruciate ligament injuries in soccer. A prospective controlled study of proprioceptive training. Knee Surgery, Sports Traumatology, Arthroscopy, 4(1): 19–21. [CrossRef]
7. Cerulli, G., Benoit, D. L., Lamontagne, M., Caraffa, A. and Liti, A. 2003. In vivo anterior cruciate ligament strain behaviour during a rapid deceleration movement: Case report. Knee Surgery, Sports Traumatology, Arthroscopy, 11(5): 307–311. [CrossRef], [Web of Science ®]
8. Chappell, J. D. and Limpisvasti, O. 2008. Effect of a neuromuscular training program on the kinetics and kinematics of jumping tasks. The American Journal of Sports Medicine, 36(6): 1081–1086. [CrossRef], [Web of Science ®]
9. Chaudhari, A. M., Hearn, B. K. and Andriacchi, T. P. 2005. Sport-dependent variations in arm position during single-limb landing influence knee loading: Implications for anterior cruciate ligament injury. The American Journal of Sports Medicine, 33(6): 824–830. [CrossRef], [PubMed], [Web of Science ®]
10. Chimera, N. J., Swanik, K. A., Swanik, C. B. and Straub, S. J. 2004. Effects of plyometric training on muscle-activation strategies and performance in female athletes. Journal of Athletic Training, 29(2): 24–31.
11. Cochrane, J. L., Lloyd, D. G., Besier, T. F., Elliott, B. C., Doyle, T. L. and Ackland, T. R. 2010. Training affects knee kinematics and kinetics in cutting maneuvers in sport. Medicine & Science in Sports & Exercise, 42(8): 1535–1544. [CrossRef], [PubMed], [Web of Science ®]
12. Cochrane, J. L., Lloyd, D. G., Buttfield, A., Seward, H. and McGivern, J. 2007. Characteristics of anterior cruciate ligament injuries in Australian football. Journal of Science and Medicine in Sport, 10(2): 96–104. [CrossRef], [Web of Science ®]
13. Dempsey, A. R., Lloyd, D. G., Elliott, B. C., Steele, J. R. and Munro, B. J. 2009. Changing sidestep cutting technique reduces knee valgus loading. The American Journal of Sports Medicine, 37(11): 2194–2200. [CrossRef], [Web of Science ®]
14. Dempsey, A. R., Lloyd, D. G., Elliott, B. C., Steele, J. R., Munro, B. J. and Russo, K. A. 2007. The effect of technique change on knee loads during sidestep cutting. Medicine & Science in Sports & Exercise, 39(10): 1765–1773. [CrossRef], [Web of Science ®]
15. Dunn, W. R. and Spindler, K. P. 2010. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): A Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. The American Journal of Sports Medicine, 38(10): 2040–2050. [CrossRef], [Web of Science ®]
16. Finch, C. F. 2006. A new framework for research leading to sports injury prevention. Journal of Science and Medicine in Sport, 9(1–2): 3–9. discussion 10 [CrossRef], [Web of Science ®]
17. Fukuda, Y., Woo, S. L., Loh, J. C., Tsuda, E., Tang, P., McMahon, P. J. and Debski, R. E. 2003. A quantitative analysis of valgus torque on the ACL: A human cadaveric study. Journal of Orthopaedic Research, 21(6): 1107–1112. [CrossRef], [Web of Science ®]
18. Gabriel, M. T., Wong, E. K., Woo, S. L., Yagi, M. and Debski, R. E. 2004. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. Journal of Orthopaedic Research, 22(1): 85–89. [CrossRef], [Web of Science ®]
19. Gianotti, S. M., Marshall, S. W., Hume, P. A. and Bunt, L. 2009. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. Journal of Science and Medicine in Sport, 12(6): 622–627. [CrossRef], [Web of Science ®]
20. Granan, L. P., Forssblad, M., Lind, M. and Engebretsen, L. 2009. The Scandinavian ACL registries 2004–2007: Baseline epidemiology. Acta Orthopaedica, 80(5): 563–567. [Taylor & Francis Online], [Web of Science ®]
21. Hashemi, J., Breighner, R., Jang, T. H., Chandrashekar, N., Ekwaro-Osire, S. and Slauterbeck, J. R. 2010. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: An in vitro simulation. Knee, 17(3): 235–241. [CrossRef], [Web of Science ®]
22. Heidt, R. S. Jr., Sweeterman, L. M., Carlonas, R. L., Traub, J. A. and Tekulve, F. X. 2000. Avoidance of soccer injuries with preseason conditioning. The American Journal of Sports Medicine, 28(5): 659–662. [Web of Science ®], [CSA]
23. Herman, D. C., Weinhold, P. S., Guskiewicz, K. M., Garrett, W. E., Yu, B. and Padua, D. A. 2008. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop-jump task. The American Journal of Sports Medicine, 36(4): 733–740. [CrossRef], [PubMed], [Web of Science ®]
24. Hewett, T. E., Lindenfeld, T. N., Riccobene, J. V. and Noyes, F. R. 1999. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. The American Journal of Sports Medicine, 27(6): 699–706. [PubMed], [Web of Science ®], [CSA]
25. Hewett, T. E., Myer, G. D., Ford, K. R., Heidt, R. S. Jr., Colosimo, A. J., McLean, S. G., van den Bogert, A. J., Paterno, M. V. and Succop, P. 2005. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. The American Journal of Sports Medicine, 33(4): 492–501. [CrossRef], [PubMed], [Web of Science ®]
26. Hewett, T. E., Stroupe, A. L., Nance, T. A. and Noyes, F. R. 1996. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. The American Journal of Sports Medicine, 24(6): 765–773. [CrossRef], [Web of Science ®], [CSA]
27. Janssen, K. W., Orchard, J. W., Driscoll, T. R. and van Mechelen, W. 2011. High incidence and costs for anterior cruciate ligament reconstructions performed in Australia from 2003-2004 to 2007–2008: Time for an anterior cruciate ligament register by Scandinavian model?. Scandinavian Journal of Medicine & Science in Sports, doi:10.1111/j.1600–0838.2010.01253.x [CrossRef]
28. Jindrich, D. L., Besier, T. F. and Lloyd, D. G. 2006. A hypothesis for the function of braking forces during running turns. Journal of Biomechanics, 39(9): 1611–1620. [CrossRef], [Web of Science ®]
29. Junge, A., Rosch, D., Peterson, L., Graf-Baumann, T. and Dvorak, J. 2002. Prevention of soccer injuries: A prospective intervention study in youth amateur players. The American Journal of Sports Medicine, 30(5): 652–659. [Web of Science ®], [CSA]
30. Kipp, K., McLean, S. G. and Palmieri-Smith, R. M. 2011. Patterns of hip flexion motion predict frontal and transverse plane knee torques during a single-leg land-and-cut maneuver. Clinical Biomechanics (Bristol, Avon), 26(5): 504–508. [CrossRef], [Web of Science ®]
31. Koga, H., Nakamae, A., Shima, Y., Iwasa, J., Myklebust, G., Engebretsen, L. … and Krosshaug, T. 2010. Mechanisms for noncontact anterior cruciate ligament injuries: Knee joint kinematics in 10 injury situations from female team handball and basketball. The American Journal of Sports Medicine, 38(11): 2218–2225. [CrossRef], [PubMed], [Web of Science ®]
32. Krosshaug, T., Nakamae, A., Boden, B. P., Engebretsen, L., Smith, G., Slauterbeck, J. R. … and Bahr, R. 2007. Mechanisms of anterior cruciate ligament injury in basketball: Video analysis of 39 cases. The American Journal of Sports Medicine, 35(3): 359–367. [CrossRef], [PubMed], [Web of Science ®]
33. Li, G., Rudy, T. W., Sakane, M., Kanamori, A., Ma, C. B. and Woo, S. L. 1999. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. Journal of Biomechanics, 32(4): 395–400. [CrossRef], [Web of Science ®], [CSA]
34. Lim, B. O., Lee, Y. S., Kim, J. G., An, K. O., Yoo, J. and Kwon, Y. H. 2009. Effects of sports injury prevention training on the biomechanical risk factors of anterior cruciate ligament injury in high school female basketball players. The American Journal of Sports Medicine, 37(9): 1728–1734. [CrossRef], [Web of Science ®]
35. Lloyd, D. G. 2001. Rationale for training programs to reduce anterior cruciate ligament injuries in Australian football. The Journal of Orthopaedic and Sports Physical Therapy, 31(11): 645–654. discussion 661 [Web of Science ®], [CSA]
36. Lloyd, D. G. and Buchanan, T. S. 1996. A model of load sharing between muscles and soft tissues at the human knee during static tasks. Journal of Biomechanical Engineering, 118(3): 367–376. [CrossRef], [Web of Science ®], [CSA]
37. Lloyd, D. G. and Buchanan, T. S. 2001. Strategies of muscular support of varus and valgus isometric loads at the human knee. Journal of Biomechanics, 34(10): 1257–1267. [CrossRef], [PubMed], [Web of Science ®], [CSA]
38. Lloyd, D. G., Buchanan, T. S. and Besier, T. F. 2005. Neuromuscular biomechanical modeling to understand knee ligament loading. Medicine & Science in Sports & Exercise, 37(11): 1939–1947. [CrossRef], [PubMed], [Web of Science ®]
39. Lyman, S., Koulouvaris, P., Sherman, S., Do, H., Mandl, L. A. and Marx, R. G. 2009. Epidemiology of anterior cruciate ligament reconstruction: Trends, readmissions, and subsequent knee surgery. The Journal of Bone and Joint Surgery. American Volume, 91(10): 2321–2328. [CrossRef], [Web of Science ®]
40. Mandelbaum, B. R., Silvers, H. J., Watanabe, D. S., Knarr, J. F., Thomas, S. D., Griffin, L. Y. … and Garrett, W. Jr. 2005. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine, 33(7): 1003–1010. [CrossRef], [Web of Science ®]
41. Markolf, K. L., Burchfield, D. M., Shapiro, M. M., Shepard, M. F., Finerman, G. A. and Slauterbeck, J. L. 1995. Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research, 13(6): 930–935. [CrossRef], [PubMed], [Web of Science ®], [CSA]
42. McGinley, J. L., Baker, R., Wolfe, R. and Morris, M. E. 2009. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture, 29(3): 360–369. [CrossRef], [PubMed], [Web of Science ®]
43. McLean, S. G., Borotikar, B. and Lucey, S. M. 2010. Lower limb muscle pre-motor time measures during a choice reaction task associate with knee abduction loads during dynamic single leg landings. Clinical Biomechanics (Bristol, Avon), 25(6): 563–569. [CrossRef], [Web of Science ®]
44. McLean, S. G., Huang, X., Su, A. and Van Den Bogert, A. J. 2004. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clinical Biomechanics (Bristol, Avon), 19(8): 828–838. [CrossRef], [Web of Science ®]
45. McLean, S. G., Huang, X. and van den Bogert, A. J. 2005. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: Implications for ACL injury. Clinical Biomechanics (Bristol, Avon), 20(8): 863–870. [CrossRef], [Web of Science ®]
46. McLean, S. G., Huang, X. and van den Bogert, A. J. 2008. Investigating isolated neuromuscular control contributions to non-contact anterior cruciate ligament injury risk via computer simulation methods. Clinical Biomechanics (Bristol, Avon), 23(7): 926–936. [CrossRef], [Web of Science ®]
47. McLean, S. G. and Samorezov, J. E. 2009. Fatigue-induced ACL injury risk stems from a degradation in central control. Medicine & Science in Sports & Exercise, 41(8): 1661–1672. [CrossRef], [Web of Science ®]
48. Meyer, E. G. and Haut, R. C. 2008. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. Journal of Biomechanics, 41(16): 3377–3383. [CrossRef], [PubMed], [Web of Science ®]
49. More, R. C., Karras, B. T., Neiman, R., Fritschy, D., Woo, S. L. and Daniel, D. M. 1993. Hamstrings—an anterior cruciate ligament protagonist. An in vitro study. The American Journal of Sports Medicine, 21(2): 231–237. [CrossRef], [Web of Science ®]
50. Myer, G. D., Ford, K. R., Brent, J. L. and Hewett, T. E. 2007. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskeletal Disorders, 8: 39 doi:1471–2474–8–39 [CrossRef], [PubMed], [Web of Science ®]
51. Myer, G. D., Ford, K. R., McLean, S. G. and Hewett, T. E. 2006. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. The American Journal of Sports Medicine, 34(3): 445–455. [CrossRef], [Web of Science ®]
52. Myer, G. D., Ford, K. R., Palumbo, J. P. and Hewett, T. E. 2005. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. Journal of Strength and Conditioning Research, 19(1): 51–60. [PubMed], [Web of Science ®]
53. Myklebust, G., Engebretsen, L., Braekken, I. H., Skjolberg, A., Olsen, O. E. and Bahr, R. 2003. Prevention of anterior cruciate ligament injuries in female team handball players: A prospective intervention study over three seasons. Clinical Journal of Sports Medicine, 13(2): 71–78. [CrossRef], [Web of Science ®], [CSA]
54. Noyes, F. R. 1977. Functional properties of knee ligaments and alterations induced by immobilization: A correlative biomechanical and histological study in primates. Clinical Orthopaedics and Related Research, Mar–Apr: 210–242. [Web of Science ®]
55. Noyes, F. R. and Grood, E. S. 1976. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. The Journal of Bone and Joint Surgery. American Volume, 58(8): 1074–1082. [Web of Science ®]
56. Oiestad, B. E., Engebretsen, L., Storheim, K. and Risberg, M. A. 2009. Knee osteoarthritis after anterior cruciate ligament injury: A systematic review. The American Journal of Sports Medicine, 37(7): 1434–1443. [CrossRef], [Web of Science ®]
57. Paterno, M. V., Schmitt, L. C., Ford, K. R., Rauh, M. J., Myer, G. D., Huang, B. and Hewett, T. E. 2010. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American Journal of Sports Medicine, 38(10): 1968–1978. [CrossRef], [PubMed], [Web of Science ®]
58. Pfeiffer, R. P., Shea, K. G., Roberts, D., Grandstrand, S. and Bond, L. 2006. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. The Journal of Bone and Joint Surgery. American Volume, 88(8): 1769–1774. [CrossRef], [Web of Science ®]
59. Rochcongar, P., Laboute, E., Jan, J. and Carling, C. 2009. Ruptures of the anterior cruciate ligament in soccer. International Journal of Sports Medicine, 30(5): 372–378. [CrossRef], [Web of Science ®]
60. Roos, H., Ornell, M., Gardsell, P., Lohmander, L. S. and Lindstrand, A. 1995. Soccer after anterior cruciate ligament injury—an incompatible combination? A national survey of incidence and risk factors and a 7-year follow-up of 310 players. Acta Orthopaedica Scandinavia, 66(2): 107–112. [Taylor & Francis Online], [CSA]
61. Shin, C. S., Chaudhari, A. M. and Andriacchi, T. P. 2011. Valgus plus internal rotation moments increase anterior cruciate ligament strain more than either alone. Medicine & Science in Sports & Exercise, 43(8): 1484–1491. [CrossRef], [Web of Science ®]
62. Simon, R. A., Everhart, J. S., Nagaraja, H. N. and Chaudhari, A. M. 2010. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. Journal of Biomechanics, 43(9): 1702–1707. [CrossRef], [Web of Science ®]
63. Soderman, K., Werner, S., Pietila, T., Engstrom, B. and Alfredson, H. 2000. Balance board training: Prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surgery, Sports Traumatology, Arthroscopy, 8(6): 356–363. [CrossRef], [Web of Science ®]
64. Steffen, K., Myklebust, G., Olsen, O. E., Holme, I. and Bahr, R. 2008. Preventing injuries in female youth football—A cluster-randomized controlled trial. Scandinavian Journal of Medicine & Science in Sports, 18(5): 605–614. [CrossRef], [Web of Science ®]
65. Wedderkopp, N., Kaltoft, M., Holm, R. and Froberg, K. 2003. Comparison of two intervention programmes in young female players in European handball—with and without ankle disc. Scandinavian Journal of Medicine & Science in Sports, 13(6): 371–375. [CrossRef], [Web of Science ®]
66. Winter, D. 2005. Motor control of human movement, 3rd, Hoboken, NJ: John Wiley & Sons, Inc.
67. Withrow, T. J., Huston, L. J., Wojtys, E. M. and Ashton-Miller, J. A. 2008. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. The Journal of Bone and Joint Surgery. American Volume, 90(4): 815–823. [CrossRef], [Web of Science ®]
68. Woo, S. L., Gomez, M. A., Sites, T. J., Newton, P. O., Orlando, C. A. and Akeson, W. H. 1987. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. The Journal of Bone and Joint Surgery. American Volume, 69(8): 1200–1211.
69. The World Bank. (2010, June). World population estimates. http://data.worldbank.org.dbgw.lis.curtin.edu.au (http://data.worldbank.org.dbgw.lis.curtin.edu.au)
70. Wu, J. L., Hosseini, A., Kozanek, M., Gadikota, H. R., Gill VI, T. J. and Li, G. 2010. Kinematics of the anterior cruciate ligament during gait. The American Journal of Sports Medicine, 38(7): 1475–1482. [CrossRef], [Web of Science ®]
71. Wu, J. L., Seon, J. K., Gadikota, H. R., Hosseini, A., Sutton, K. M., Gill, T. J. and Li, G. 2010. In situ forces in the anteromedial and posterolateral bundles of the anterior cruciate ligament under simulated functional loading conditions. The American Journal of Sports Medicine, 38(3): 558–563. [CrossRef], [Web of Science ®]
72. Zazulak, B. T., Hewett, T. E., Reeves, N. P., Goldberg, B. and Cholewicki, J. 2007. Deficits in neuromuscular control of the trunk predict knee injury risk: A prospective biomechanical-epidemiologic study. The American Journal of Sports Medicine, 35(7): 1123–1130. [CrossRef], [PubMed], [Web of Science ®]
73. Zebis, M. K., Bencke, J., Andersen, L. L., Dossing, S., Alkjaer, T., Magnusson, S. P. … and Aagaard, P. 2008. The effects of neuromuscular training on knee joint motor control during sidecutting in female elite soccer and handball players. Clinical Journal of Sports Medicine, 18(4): 329–337. [CrossRef], [Web of Science ®]


.jpg)